
The Mössbauer spectra revealed the disordered atomic environment. Therefore, the empirical formula of aluminium oxide is Al 2O 3. 5 Citations Metrics Abstract Single crystals of -Al 2 O 3 and LiNbO 3 were implanted with 57 Co (dose: up to 2×10 15 atoms/cm 2) and with 57 Fe (dose: 2×10 15 atoms/cm 2) ions.
Each oxide has a charge of 2-, which means that we can get 6 negative charges by having 3 oxides. Each aluminium has a charge of 3+, which means that we can get 6 positive charges by having 2 aluminiums. Therefore Induced Emf e N t ve sing is due to Lenz s Law Example A coil with an average diameter of 0. This means that we can balance the charge of this ionic compound by having 6 positive charges and 6 negative charges. A strong magnetic field is applied on a direction of the field.
The first number that 3 and 2 go into is 6 (the LCM is 6).
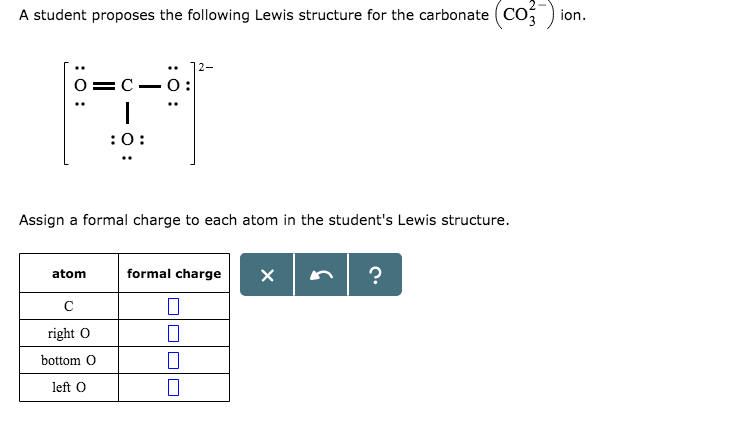
The balancing for aluminium oxide is slightly different because the positive and negative charges do not go into each other the 3 positive charge from aluminium and the 2 negative charge from oxygen do not go into each other. The overall charge for an ionic compound must be 0, which means that the charges from aluminium and oxygen must balance out. Oxygen is in group 6 in the periodic table, which means that it will gain 2 electrons resulting in it having a 2 negative charge (O 2-). Aluminium is a metal and is in group 3 in the periodic table, which means that it will lose 3 electrons resulting in it having a 3 positive charge (Al 3+). In order to calculate the formal charges for CO3 2- well use the equation:Formal charge of valence electrons - nonbonding val electrons - bonding e. You can effortlessly find every single detail about the elements from this single Interactive Periodic table.What is the empirical formula of aluminium oxide?Īluminium oxide is made out of aluminium and oxygen atoms. Let me tell you how this Interactive Periodic Table will help you in your studies.ġ).
CO IRON CHARGE FREE
Elements Common Charges 1 Charge of Hydrogenionġ+ 2 Charge of Heliumion 0 3 Charge of Lithiumionġ- 10 Charge of Neonion 0 11 Charge of Sodiumionġ- 18 Charge of Argonion 0 19 Charge of PotassiumionĢ+, 3+, 4+, 5+ 24 Charges of Chromiumions 2+, 3+,6+ 25 Charges of Manganeseions 2+, 4+, 7+ 26 Charges of Ironionsģ+ 32 Charges of Germaniumions 4-, 2+, 4+ 33 Charges of Arsenicions 3-, 3+, 5+ 34 Charges of Seleniumions 2-, 4+, 6+ 35 Charges of Bromineionsġ-, 1+, 5+ 36 Charge of Kryptonion 0 37 Charge of Rubidiumion 1+ 38 Charge of Strontiumion 2+ 39 Charge of Yttriumion 3+ 40 Charge of Zirconiumion 4+ 41 Charges of Niobiumions 3+, 5+ 42 Charges of Molybdenumions 3+, 6+ 43 Charge of Technetiumion 6+ 44 Charges of Rutheniumions 3+, 4+, 8+ 45 Charge of Rhodiumion 4+ 46 Charges of Palladiumions 2+, 4+ 47 Charge of Silverionġ+ 48 Charge of Cadmiumion 2+ 49 Charge of Indiumion 3+ 50 Charges of Tinions 2+, 4+ 51 Charges of Antimonyions 3-, 3+, 5+ 52 Charges of Telluriumions 2-, 4+, 6+ 53 Charge of Iodineionġ- 54 Charge of Xenonion 0 55 Charge of Cesiumionġ+ 56 Charge of Bariumion 2+ 57 Charge of Lanthanumion 3+ 58 Charges of Ceriumions 3+, 4+ 59 Charge of Praseodymiumion 3+ 60 Charges of Neodymiumions 3+, 4+ 61 Charge of Promethiumion 3+ 62 Charge of Samariumion 3+ 63 Charge of Europiumion 3+ 64 Charge of Gadoliniumion 3+ 65 Charges of Terbiumions 3+, 4+ 66 Charge of Dysprosiumion 3+ 67 Charge of Holmiumion 3+ 68 Charge of Erbiumion 3+ 69 Charge of Thuliumion 3+ 70 Charge of Ytterbiumion 3+ 71 Charge of Lutetiumion 3+ 72 Charge of Hafniumion 4+ 73 Charge of Tantalumion 5+ 74 Charge of Tungstenion 6+ 75 Charges of Rheniumions 2+, 4+, 6+, 7+ 76 Charges of Osmiumions 3+, 4+, 6+, 8+ 77 Charges of Iridiumions 3+, 4+, 6+ 78 Charges of Platinumions 2+, 4+, 6+ 79 Charges of Goldions 1+, 2+, 3+ 80 Charges of Mercuryions 1+, 2+ 81 Charges of Thalliumions 1+, 3+ 82 Charges of Leadions 2+, 4+ 83 Charge of Bismuthion 3+ 84 Charges of Poloniumions 2+, 4+ 85 Charge of Astatineion Unknown 86 Charge of Radonion 0 87 Charge of Franciumion Unknown 88 Charge of Radiumion 2+ 89 Charge of Actiniumion 3+ 90 Charge of Thoriumion 4+ 91 Charge of Protactiniumion 5+ 92 Charges of Uranium ions 3+, 4+, 6+ Free Gift for you: Interactive Periodic Table List of elements with their common ionic charges are mentioned below.Įlements with multiple ionic charges are also mentioned in this table. the resultant TFC membranes exhibited a CO 2 permeance of 1990 GPU with a CO 2 /N 2. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. 2 Such a selection may be done simply by the ion charge (valence. This electric charge generated on the ion is known as Ionic charge. Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).


 0 kommentar(er)
0 kommentar(er)
